Product Cat. No.: GBS-059
For Research Use Only.
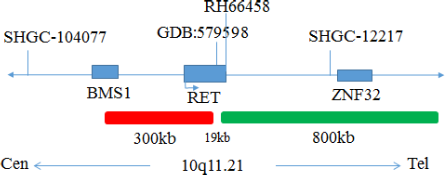
RET(10q11) gene break apart probe reagent
10 Tests/box
This kit uses Orange fluorescein and Green fluorescein labeled RET, to combine RET genes with the target site by in situ hybridization.
The kit consists of RET dual color probe as shown in Table 1.
| Component name | Specifications | Quantity | Main components |
|---|---|---|---|
| RET dual-color probe | 100μL/Tube | 1 | RET Orange probe ; RET Green probe |
Keep sealed away from light at -20oC±5oC, and the validity period is 12 months. After the cover is opened, it can be sealed and stored in 2~8°C away from light within 24 hours. After the cover is opened, it should be sealed and stored in -20±5°C away from light for a long time. Transport with temperature below 0°C.
Fluorescence microscopy imaging systems, including fluorescence microscopy and filter sets suitable for DAPI (367/452), Green (495/517),and Orange (547/565).
1. Applicable specimen types: Paraffin-embedded specimens for surgical resection or biopsy.
2. Tissue should be fixed with 4% neutral formaldehyde fixation solution within 1 hour after in vitro, and the tissue should be fixed by conventional dehydration and paraffin embedding.
1. Pre-hybridization or Pretreatment
Baking: Slides heating at 80oC for 30min or 65oC for 2h or overnight.
Dewaxing: According to the customer laboratory protocol (Commonly with Xylene for 15min).
Hydration: Take out the slides and put them respectively into 100%, 85% and 70% EtOH at room temperature for 3 minutes each.
Take out the slides, and immerse them in deionized water for 3 minutes. Remove the excess of water on the slides by air-drying.
Permeation: Immerse the slides in deionized water at 100oC and boil continuously for 20-40 minutes (Conventional 20min). Remove the excess of water on the slides by air-drying.
Digestion: Protease enzymic digestion at 37°C for 10-40 minutes. Mix the protease work buffer (50mmol HCl) and the 10x protease solution (Pepsin concentration 5%) in a proportion of 9:1 to prepare the enzymatic digestion solution.
Washing: Wash with 2xSSC at room temperature for 5 minutes.
Dehydration: Take out the slides and dehydrate in 70%, 85%, and 100% gradient ethanol at room temperature for 2 minutes each time.Remove the excess of EtOH solution on the slides by air-drying.
2. Denaturation and Hybridization
The following operations need to be carried out in the darkroom.
3. Washing
The following operations need to be carried out in the darkroom.
4. Counterstaining
The following operations should be performed in a darkroom
10μL DAPI compound dye is dropped in the hybridization area of the glass slide and immediately covered. The suitable filter is selected for glass slide observation under the fluorescence microscope.
5. FISH results observation
Place the counterstained film under the fluorescence microscope, and first put it under the low-power objective lens (10x) confirm the cell area under the microscope; Go to 40x under the objective lens, find a position where the cells are evenly distributed; Then in the high-power objective (100x) the FISH results of nuclei were observed.

Negative: 2 fusion

Positive: 1 orange 1 green 1 fusion
1.The results of this kit will be affected by various factors of the sample itself, but also limited by hybridization temperature and time, operating environment and the limitations of current molecular biology technology, which may lead to wrong results.
2.Users must understand the potential errors and accuracy limitations that may exist in the detection process.
3.All chemicals are potentially dangerous. Avoid direct contact. Used kits are clinical waste and should be properly disposed off.
V1. 0: Approval date: September 3, 2019.
V1. 2: Revision date: December 7, 2021.
