Storage conditions & Validity
Keep sealed away from light at -20oC±5oC. The product is valid for 12 months. Avoid unnecessary repeated freezing and thawing that should not exceed 10 times. After opening, within 24 hours for short-term preservation, keep sealed at 2-8oC in dark. For long-term preservation after opening, keep the lid sealed at -20oC±5oC away from light. The kit is transported below 0oC.
Applicable Instruments
Fluorescence microscopy imaging systems, including fluorescence microscopy and filter sets suitable for DAPI (367/452), Green (495/517),and Orange (547/565).
Sample Requirements
Tissue samples:
1. Applicable specimen type: paraffin embedded specimen of surgical resection or biopsy tissue.
2. The tissue should be fixed with 4% neutral formaldehyde fixation solution within 1 hour after in vitro. After tissue fixation, it should be regularly dehydrated and embedded in paraffin.
Cell sample:
1. Applicable specimen type: unfixed fresh bone marrow specimen (stored at 2-8°C for no more than 24 hours).
2. Sample collection take 1-3ml of bone marrow cells anticoagulated with heparin sodium.
3. Sample preservation: after fixation, the cell suspension shall be stored at -20±5 °C for no more than 12 months; The prepared cell slides can be stored at -20±5 °C for no more than 1 month. When the sample storage temperature is too high or too low, or the cell suspension is volatilized excessively or polluted during storage, the sample will not be used for detection.
Related Reagents
The following reagents are required for the experiment but not provided in this kit
- 20×SSC, pH 5.3±0.2
Weigh 176g of sodium chloride and 88g of sodium citrate, dissolve in 800mL of deionized water, adjust the pH to 5.3±0.2 at room temperature, and complete to 1 L with deionized water. High-pressure steam sterilization, stored at 2-8oC,the solution shelf life is of 6 months. Discard if the reagent appears cloudy (turbid) or contaminated.
- 2×SSC, pH 7.0±0.2
Take 100mL of the above 20xSSC, dilute with 800mL deionized water, mix, adjust the pH to 7.0±0.2 at room temperature, complete to 1L with deionized water, stored at 2-8oC, the shelf life is of 6 months. Discard if the reagent appears cloudy (turbid) or contaminated.
- Ethanol Solution: 70% ethanol, 85% ethanol
Dilute 700ml, 850ml of ethanol with deionized water to 1L. The shelf life is of 6 months. Discard if the reagent appears cloudy (turbid) or contaminated.
- 0.3% NP-40/0.4xSSC solution, pH 7.0-7.5
Take 0.6mL NP-40 and 4mL 20×SSC, add 150mL deionized water, mix, adjust the pH to 7.0-7.5 at room temperature, with deionized water complete to a volume of 200mL. Stored at 2-8oC, the shelf life is of 6 months. Discard if the reagent appears cloudy (turbid) or contaminated.
- Fixation solution (methanol: glacial acetic acid = 3:1)
Prepare a ready to use fixation solution by mixing thoroughly 30ml of methanol and 10ml of glacial acetic acid.
- 0.075M KClsolution
Weigh 2.8g of potassium chloride, dissolve in 400mL of deionized water and complete to 500mL with deionized water. Stored at room temperature, the solution shelf life is of 6 months. Discard if the reagent appears cloudy (turbid) or contaminated.
- Diamidinyl phenylindole (DAPI) counterstain
Use commercially available anti-quenching DAPI counterstain.
Instruction
1. Sample processing before hybridization
Tissue samples:
Baking: Slides heating at 80oC for 30min or 65oC for 2h or overnight.
Dewaxing: According to the customer laboratory protocol (Commonly with Xylene for 15min).
Hydration: Take out the slides and put them respectively into 100%, 85% and 70% EtOH at room temperature for 3 minutes each.Take out the slides, and immerse them in deionized water for 3 minutes. Remove the excess of water on the slides by air-drying.
Permeation: Immerse the slides in deionized water at 100oC and boil continuously for 20-40 minutes (Conventional 20min). Remove the excess of water on the slides by air-drying.
Digestion: Protease enzymic digestion at 37°C for 10-40 minutes. Mix the protease work buffer (50mmol HCl) and the 10x protease solution (Pepsin concentration 5%) in a proportion of 9:1 to prepare the enzymatic digestion solution.
Washing: Wash with 2xSSC at room temperature for 5 minutes.
Dehydration: Take out the slides and dehydrate in 70%, 85%, and 100% gradient ethanol at room temperature for 2 minutes each time.Remove the excess of EtOH solution on the slides by air-drying.
Cell sample:
- Sample collection: Take 3mL of anticoagulated bone marrow cell samples.
- Cell harvesting: Place 3 mL of anticoagulated peripheral blood in a 15 mL centrifuge tube, centrifuge at 500g for 5 min, carefully discard the supernatant, and resuspend about 500μL of the residue.
- Cell washing: Add 5 mL of 1×PBS buffer, mix and resuspend the cell pellet, centrifuge at 500g for 5 min, carefully discard the supernatant,and resuspend the cells with about 500μL of the residue; repeat 1 time.
- Cells hypotonicty: Add 10mL of hypotonic solution pre-warmed to 37oC and place in an water bath at 37oC for 15-20min.
- Cells pre-fixation: Pre-fix the cells by adding 1mL (10% by volume) of fixative solution to the cell suspension after the completion of hypotonic osmosis. Gently pipette, mix and centrifuge for 5 min at 500g, discard the supernatant, and resuspend about 500μL of the residue.
- Cell fixation: Slowly add 10mL of fixative solution to the cell suspension at room temperature for 10 min, centrifuge at 500g for 5 min,and resuspend the cells with about 500μL of the residue; repeat once (the cells may be fixed several times until the cells pellet is washed and cleaned).
- Cell suspension preparation: Pipet the supernatant and add the appropriate amount of fixative solution to prepare the appropriate cell suspension concentration.
- Slides preparation: Pipet 3-5μl of cell suspension drop onto the slides, put at 56oC for 30min.
- Pretreatment: At room temperature, rinse the glass slides twice with 2xSSC (pH 7.0) solution for 5min each time.
- Dehydration: Place the glass slides in 70% ethanol, 85% ethanol and 100% ethanol and dry for 2 minutes.
2. Denaturation and Hybridization
The following operations should be performed in a darkroom.
Tissue samples:
- Take out the probe put at room temperature for 5min. Mix and centrifuge briefly. Take 10μl droplet in the cell and drop in the hybridization zone, immediately cover 22mmx22mm glass slide area; spread evenly without bubbles the probe under the glass slide covered area and seal edges with rubber (edge sealing must be thorough to prevent dry film from affecting the test results during hybridization).
- Place the glass slides in the hybridization instrument, denature at 85°C for 5 minutes (the hybridizer should be preheated to 85oC) and hybridize at 42°C for 2 to 16 hours.
Cell sample:
- Take out the probe put at room temperature for 5min. Mix and centrifuge briefly. Take 10μl droplet in the cell and drop in the hybridization zone, immediately cover 22mmx22mm glass slide area;spread evenly without bubbles the probe under the glass slide covered area and seal edges with rubber (edge sealing must be thorough to prevent dry film from affecting the test results during hybridization).
- Place the glass slides in the hybridization instrument, denature at 88°C for 2 minutes (the hybridizer should be preheated to 88oC) and hybridize at 45°C for 2 to 16 hours.
3. Washing
The following operations should be performed in a darkroom.
- Take out the hybridized glass slides, remove the rubber on the coverslip and immediately immerse the slides in a 2xSSC solution for 5 seconds and remove the coverslip.
- Place the slides in a 2×SSC at room temperature for 1 min.
- Take out the slides and immerse in a preheated at 68°C 0.3% NP-40/0.4xSSC (Preparation of 0.3% NP-40/0.4xSSC: For 1L preparation,take 3mL NP-40 and 20mL 20xSSC, dissolve fully, mix well, and use 1M NaOH to adjust the pH to 7.2) solution and wash for 2min.
- Remove the slides and immerse in a 37°C preheated deionized water, wash for 1min and dry the slides naturally in the dark.
4. Counterstaining
The following operations should be performed in a darkroom
10μl DAPI compound dye is dropped in the hybridization area of the glass slide and immediately covered. The suitable filter is selected for glass slide observation under the fluorescence microscope.
5. FISH results observation
Put the counterstained cell drops under the fluorescence microscope, first under the low-power objective lens (10x) confirm the cell area under the microscope; Go to 40x under the objective lens, find a position where the cells are evenly distributed; Then in the high-power objective (100x) the FISH results of nuclei are observed.
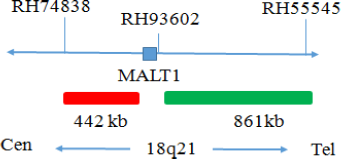
Common Signal Type Interpretation
MALT1 gene site 5 signal
MALT1 gene site 3 signal
Positive: 1 orange 1 green 1 fusion
Precautions
1. The results of this kit will be affected by various factors of the sample itself, but also limited by hybridization temperature and time,operating environment and the limitations of current molecular biology technology, which may lead to wrong results.
2.Users must understand the potential errors and accuracy limitations that may exist in the detection process.
3.All chemicals are potentially dangerous. Avoid direct contact. Used kits are clinical waste and should be properly disposed off.
Manual Approval date & Revision date
V1. 0: Approval date: May 18, 2020.
V1. 2: Revision date: December 7, 2021.



