Product Cat. No.: GBS-331
For Research Use Only.
NF1 gene deletion probe reagent
10 Tests/box
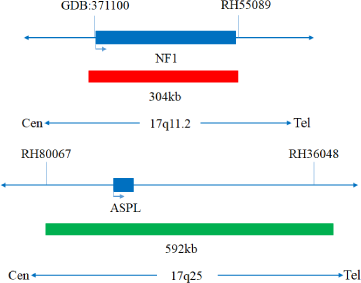
The kit uses orange fluorescein-labeled NF1 probe and green fluorescein-labeled ASPL probe to bind the NF1/ASPL probe to the target detection site by in situ hybridization.
The kit consists of NF1/ASPL dual-color probe, as shown in Table 1.
| Component name | Specifications | Quantity | Main components |
|---|---|---|---|
| NF1/ASPL dual-color probe | 100μL/Tube | 1 | NF1 orange probe ; ASPL green probe |
Keep sealed away from light at -20°C±5°C,and the validity period is 12 months.After the cover is opened, it can be sealed and stored in 2~8°C away from light within 24 hours. After the cover is opened, it should be sealed and stored in -20±5°C away from light for a long time. Transport with temperature below 0°C.
Fluorescence microscopy imaging systems,including fluorescence microscopy and filter sets suitable for DAPI (367/452),Green (495/517),and Orange (547/565).
Cell:
1. Take 1-3mL of heparin sodium anticoagulant bone marrow cells.
2. Sample preservation: Fresh bone marrow specimen without fixation (preserved at 2-8°C for no more than 24 hours). After fixation, the cell suspension can be preserved at -20±5°C for no more than 12 months; the prepared cell slide can be preserved at -20±5°C for no more than 1 month. When the storage temperature of the sample is too high or too low, the cell suspension is volatilized excessively or polluted,the sample cannot be used for detection.
Tissue:
1. Applicable specimen types: Paraffin embedded specimens from surgical excision or biopsy.
2. The tissue should be fixed with 4% neutral formaldehyde solution within 1 hour after isolation. After tissue fixation, it is routinely dehydrated and embedded in paraffin.
1. 20×SSC, pH 5.3±0.2
Weigh 176g of sodium chloride and 88g of sodium citrate, dissolve in 800mL of deionized water, adjust the pH to 5.3±0.2 at room temperature, and complete to 1L with deionized water. High-pressure steam sterilization, stored at 2-8oC, the solution shelf life is of 6 months. Discard if the reagent appears cloudy (turbid) or contaminated.
2. 2×SSC, pH 7.0±0.2
Take 100mL of the above 20xSSC, dilute with 800mL deionized water, mix, adjust the pH to 7.0±0.2 at room temperature, complete to 1L with deionized water, stored at 2-8oC, the shelf life is of 6 months. Discard if the reagent appears cloudy (turbid) or contaminated.
3. Ethanol Solution: 70% ethanol, 85% ethanol
Dilute 700ml, 850ml of ethanol with deionized water to 1L. The shelf life is of 6 months. Discard if the reagent appears cloudy (turbid) or contaminated.
4. 0.3% NP-40/0.4xSSC solution, pH 7.0-7.5
Take 0.6mL NP-40 and 4mL 20×SSC, add 150mL deionized water, mix, adjust the pH to 7.0-7.5 at room temperature, with deionized water complete to a volume of 200mL. Stored at 2-8oC, the shelf life is of 6 months. Discard if the reagent appears cloudy (turbid) or contaminated.
5. Fixation solution (methanol: glacial acetic acid = 3:1)
Prepare a ready to use fixation solution by mixing thoroughly 30ml of methanol and 10ml of glacial acetic acid.
6. 0.075M KCl solution
Weigh 2.8g of potassium chloride, dissolve in 400mL of deionized water and complete to 500mL with deionized water. Stored at room temperature, the solution shelf life is of 6 months. Discard if the reagent appears cloudy (turbid) or contaminated.
7. Diamidinyl phenylindole (DAPI) counterstain
Use commercially available anti-quenching DAPI counterstain.
1. Sample processing before hybridization:
Cells sample:
Tissue sample:
Baking: Slides heating at 80°C for 30min or 65°C for 2h or overnight.
Dewaxing: According to the customer laboratory protocol (Commonly with Xylene for 15min).
Hydration: Take out the slides and put them respectively into 100%, 85% and 70% EtOH at room temperature for 3 minutes each.
Take out the slides, and immerse them in deionized water for 3 minutes. Remove the excess of water on the slides by air-drying.
Permeation: Immerse the slides in deionized water at 100°C and boil continuously for 20-40 minutes (Conventional 20min). Remove the excess of water on the slides by air-drying.
Digestion: Protease enzymic digestion at 37°C for 10-40 minutes. Mix the protease work buffer (50mmol HCl) and the 10x protease solution (Pepsin concentration 5%) in a proportion of 9:1 to prepare the enzymatic digestion solution.
Washing: Wash with 2xSSC at room temperature for 5 minutes.
Dehydration: Take out the slides and dehydrate in 70%, 85%, and 100% gradient ethanol at room temperature for 2 minutes each time.
Remove the excess of EtOH solution on the slides by air-drying.
2. Denaturation and Hybridization
The following operations need to be carried out in the darkroom.
Cell sample:
Tissue samples:
3. Washing
The following operations need to be carried out in the darkroom.
4. Counterstaining
The following operations should be performed in a darkroom
10μl DAPI compound dye is dropped in the hybridization area of the glass slide and immediately covered. The suitable filter is selected for glass slide observation under the fluorescence microscope.
5. FISH results observation
Place the counterstained film under the fluorescence microscope, and first put it under the low-power objective lens (10x) confirm the cell area under the microscope; Go to 40x under the objective lens, find a position where the cells are evenly distributed; Then in the high-power objective (100x) the FISH results of nuclei are observed.

Negative: 2 orange 2 green

Positive: 1 orange 2 green
1.The results of this kit will be affected by various factors of the sample itself, but also limited by hybridization temperature and time,operating environment and the limitations of current molecular biology technology, which may lead to wrong results.
2.Users must understand the potential errors and accuracy limitations that may exist in the detection process.
3.All chemicals are potentially dangerous.Avoid direct contact. Used kits are waste and should be properly disposed off.
V1. 0: Approval date: July 05, 2021.
V1. 2: Revision date: September 21, 2022.
