Product Cat. No.: GBS-043
For Research Use Only
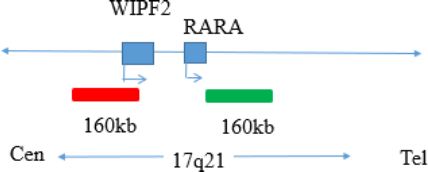
RARA gene break apart probe reagent.
10 Tests/box
This kit uses orange fluorescein and green fluorescein to label the RARA probe. The RARA probe can be combined with the target detection site by in situ hybridization.
The kit consists of RARA dual color probe as shown in Table 1.
| Component name | Specifications | Quantity | Main components |
|---|---|---|---|
| RARA dual color probe | 100μL/Tube | 1 | RARA orange probe ; RARA green probe |
Keep sealed away from light at -20oC±5oC. The product is valid for 12 months. Avoid unnecessary repeated freezing and thawing that should not exceed 10 times. After opening, within 24 hours for short-term preservation, keep sealed at 2-8oC in dark. For long-term preservation after opening, keep the lid sealed at -20oC±5oC away from light.
Fluorescence microscope imaging system, including fluorescence microscope and filtersetsuitable for DAPI (367/452), green (495/517) and orange (547/ 565).

Negative : 2 fusion

Positive : 1 orange , 1 green , 1 fusion
V1. 0: Approval date: June 1, 2020.
V1. 4: Revision date: December 7, 2021.
